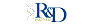Average salary: $212,500 /yearly
More stats ...therapies in oncology, as well as other research areas centered around rare diseases and immune disorders.
Summary
The Manager, Regulatory Affairs – Regulatory Project Management (RPM) provides planning and coordination supports for global and US regulatory activities...
Suggested
Work experience placement
...Responsible for leading and developing global regulatory strategy for assigned development... ...company as needed.
Responsibilities
Manages, directs, and drives the strategy for... ...5 years of experience within Regulatory Affairs
~ Knowledge of IND, NDA/BLA submission...
Suggested
...role will create and implement effective regulatory strategies to deliver on efficient and... ...provide regulatory support and guidance and manage day-to-day regulatory activities.... ...required.
~8 or More Years in regulatory affairs
~ Experience in providing regulatory strategic...
Suggested
...Leadership responsibility for global regulatory strategy to support the development of biomarkers... ...RA specific inputs into CDx Risk Management plans for individual projects/indications... ...including experience within Regulatory Affairs
~ Understanding of scientific content...
Suggested
Local area
...well as other research areas centered around rare diseases and immune disorders.
Summary
The Regulatory Data Manager will be a key member of the Global Regulatory Affairs (GRA) Process Excellence function, responsible for driving data governance, reporting, and...
Suggested
...as the GRA labeling expert to the Global Regulatory Team (GRT) and independently provides... ...for late stage projects. This position manages multiple assigned projects of increased... ...~4 or More Years of direct regulatory affairs experience, including US labeling experience...
Suggested
Local area
...This position reports to the Functional Area Head, Global Regulatory Affairs. The incumbent responsible for supporting the development and... ...teams to prepare, review and submit regulatory filing documents, manage documentation systems and maintain interactions with...
Suggested
Full time
Contract work
...degree of quality in the execution of all Global Oncology Medical Affairs (GOMA) Company Sponsored Studies (CSS) and Medical Access/... ...Accountable for accuracy and timeliness of information in all data management activities, databases and tracking systems.Company Sponsored...
Suggested
Contract work
...with disabilities.
Summary
This position leads the Global Regulatory Affairs (GRA) Global Labeling function, combining knowledge of... ...collaborators/alliance partners. They will also develop, oversee, and manage implementation of global labeling development and quality...
Suggested
For contractors
Local area
...This role is responsible for leading the Regulatory Writing function, overseeing, directing... ...This will include resolving conflicts, managing and influencing global stakeholders across... ...such as Clinical, QCP, BDM, Regulatory Affairs and Medical Affairs in all aspects of regulatory...
Suggested
Contract work
For contractors
...Director, Head of Global Dossier Planning, Regulatory Operations is responsible for leading... ...dossier planning/submission topics.
Manages and develops internal talent ensuring a... ...required.
~4 or More Years regulatory affairs including filing NDA and sNDA. BLA filing...
Suggested
...leading and mentoring a dynamic and global Regulatory Intelligence & Policy team, through direct and indirect line management, to identify and lead global corporate initiatives... ...will also work with the Global Regulatory Affairs Leadership Team (GRA-LT) to define DS'...
Suggested
Temporary work
Work alone
...disorders.
Summary The Senior Director, Global Oncology Medical Affairs is responsible for developing the Global Medical Affairs (GMA)... ...in the GMA plan including Launch Readiness and Life Cycle Management for the assigned compound and will report into the Global...
Suggested
Local area
...Job Description
R&D Partners is seeking to hire a Global Regulatory CMC Manager in Lawrenceville, NJ .
Your main responsibilities... ...cross-functional development/commercial teams and Regulatory Affairs teams.
What we are looking for in a Global Regulatory...
Suggested
...for the execution of activities in support of Global Medical Affairs Scientific Engagement strategies and plan for the Solid Tumor... ...External Experts (KEE) engagement strategy and operational plan. Management and updating of KEE mapping and engagement plans, and related...
Suggested
Local area
Work alone
...other research areas centered around rare diseases and immune disorders.
Summary
The Senior Director, Global Oncology Medical Affairs, Evidence Generation role is responsible for co-leading a cross-functional Evidence Generation Team and developing an Integrated...
...aspects of statistical activities; collaborates closely with data manager to ensure high quality data. Work closely with internal... ...phase of the development are scientifically sound, can fulfill regulatory requirements and deliver the pre-specified product profile....
Contract work
...including veterans and people with disabilities.
Summary
The Manager External Data Management, is accountable for an end-to-end... ..., companion diagnostics trials, clinical development, and regulatory submissions. The position will perform validation of the electronically...
Contract work
Work experience placement
...centered around rare diseases and immune disorders.
Summary
Manage Global DX Computer System Validation (CSV) projects within... ...activities, assuring that these systems are compliant with global regulatory and Daiichi Sankyo requirements, and business expectations....
For contractors
...as well as other research areas centered around rare diseases and immune disorders.
Summary
The Manager, Medical Training will support Global Oncology Medical Affairs (GOMA) TA/franchise in developing and executing global high-quality training programs and ensuring...
...Summary
The Director, Global eQMS Management QA GMP, reports to the Senior Director,... ...evolving needs of the organization and regulatory requirements. Drive collaboration, alignment... ...related functions (Supply Chain, Regulatory Affairs, CMC, Pharmaceutical Technology) who are...
...deliverable quality, and expedite the preparation of oncology compound regulatory submission. It will also to maintain institutional knowledge... ...resource planning, work in tandem with Biostatistics and Data Management members to ensure best vendor performance, monitor analysis...
For contractors
...Corporate Communications function responsible for planning and managing global external and internal communications across... ...limited investor relations, RD, global and US medical affairs and marketing, legal, regulatory and congress planning Qualifications: Successful...
...around rare diseases and immune disorders.
Summary
The Manager of Data Governance Stewardship will work in areas required for... ...understanding of key industry best practices as well as legislative, regulatory and industry artifacts / frameworks, such as, the following: o...
...disabilities.
Summary
The Associate Director, External Data Management, is accountable for the end-to-end delivery of External data... ...trials, companion diagnostics trials, clinical development, and regulatory submissions. This position may propose strategies for external...
Contract work
...This position supports proactive safety surveillance and risk management for assigned clinical studies in partnership with Clinical Safety... ...Sheet (CCDS), Risk Management Plan(s) (RMPs) - Contributes to regulatory authority requests or communication for assigned clinical...
...as well as other research areas centered around rare diseases and immune disorders.
Summary
The primary responsibilities of the Manager, Omnichannel Marketing is to support the development, planning and implementation of omnichannel marketing to key customer segments...
...quality. This position requires working knowledge of clinical data management processes, Electronic Data Capture (EDC)/related applications,... ...standards, Good Clinical Practices (GCPs), applicable regulatory requirements, and other relevant guidelines. Serve as a lead to...
Work experience placement
Shift work
...Innova Solutions is immediately hiring for a Revenue Program Manager
Position type: Contract
Duration: 12+ Months
Location: Remote
Description:
As a RevenueProgram Manager you will :-...
Hourly pay
Contract work
Temporary work
Work experience placement
Immediate start
Remote job
Worldwide
Flexible hours
**Sales Manager**
at Offensive Security Holmdel, New Jersey Offensive Security (OffSec) is the worlds most trusted provider of cyber... ...* help defend us against any claims.
* comply with legal or regulatory requirements.
**Our legal basis for processing your...
Contract work
For contractors