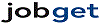Average salary: $190,000 /yearly
More statsSearch Results: 18,765 vacancies
...The Role
Moderna is seeking a highly skilled and motivated Scientist or Engineer to join our Respiratory Drug Product Development (DPD) team in Norwood, MA. To support Moderna's exciting pipeline of products, the DPD team works to develop, characterize, and implement...
Suggested
Holiday work
Flexible hours
...is made between project teams driving asset (molecule) strategy development and high level execution and those driving indication strategy... ...from clinical operational to more commercial orientation.
The Drug Development Project Manager (DDPM) oversees the high-level...
Suggested
Shift work
...patient story, and together, we are writing a new chapter in genomic medicines.
Reporting to our Senior Director, Drug Product, Process Development, we are seeking an Associate Director to join our team. This position is predominantly a lab-based role and will be responsible...
Suggested
...Job Details
The Role:
Moderna is seeking a highly skilled and motivated Scientist or Senior Engineer to join our Drug Product Development (DPD) team in Norwood, MA. To support Modernas exciting pipeline of products, the DPD team works to develop, characterize, and...
Suggested
Holiday work
Flexible hours
...Biogen is presently looking to fill a Scientist II position within its Anti-Sense Oligonucleotide (ASO) Formulation and Drug Product Development group. The candidate will be responsible for formulation and process development activities of oligonucleotide drug product...
Suggested
Summer work
Local area
...level.
Work with the project team to develop the integrated development plan.
Enable transparent and candid communication across the... ...PhD, Master's or BS/BA/MBA degree
~8+ years' experience in a drug discovery development field or scientific, functional experience...
Suggested
Immediate start
...patients through the advancement of our cutting-edge mRNA technology! About the Opportunity
As a Process Development Engineer/ Scientist in the Global Drug Substance Development group you will focus on CMC development, optimization and scale-up of mRNA synthesis and...
Suggested
~ A clinical degree (e.g., RPh/PharmD, BSN, RN)
~3-5 years drug safety or clinical safety experience
~ Two years clinical experience in a medical setting, demonstrate general medical and pharmacology knowledge
by Jobble
Suggested
...the world.
The Role:
Reporting to the Vice President of Manufacturing and CMC, the Associate Director/Director of Drug Substance Development will be responsible for all aspects of drug substance development across multiple Cardurion programs. The Associate...
Suggested
Holiday work
Temporary work
Immediate start
Flexible hours
...The Role:
Moderna is seeking a Project Manager within the Drug Substance Business Unit. This position is responsible to drive a... ...of 8 years supporting manufacturing operations and/or process development in a regulated environment.
* Specific Certifications or Training...
Suggested
Holiday work
Flexible hours
...serious retinal disease
* advancing best-in-class proprietary drug delivery technologies
* innovative pipeline of sustained... ...Based in Watertown, MA and reporting to the Director, Formulation Development, this position is responsible for leading late-stage ocular drug...
Suggested
Remote job
Flexible hours
...transformative medicine to patients through the advancement of our cutting-edge mRNA technology!
Our Team:
The drug substance development team at Waltham MA is composed of a group of scientists, engineers, and contractors with diverse backgrounds. We design,...
Suggested
For contractors
Internship
Remote job
Flexible hours
...Responsibilities
Job title: Intern: mRNA Drug Substance Process Development
Location : Waltham, MA
Remote working: N/A, and Limited of travel expected:
Job type : Intern
In the race for the future of health – The Sanofi mRNA Center of Excellence (...
Suggested
For contractors
Internship
Remote job
Flexible hours
...represents the most advanced potassium channel modulator in clinical development for multiple indications. Building upon the positive results... ...programs that leverage our extensive ion channel expertise and drug discovery capabilities to identify validated drug targets and...
Suggested
Temporary work
Local area
...Job Title: Senior Drug Safety Specialist
Location: Cambridge, MA
Duration: 8+ Months
Job Description:
Duties:
Reporting... ...records management and compliance across the clinical development and marketing programs. You will be a key contributor to the day...
Suggested
Business Development Intern
LifeMine is pioneering a bold new approach, Top-Down Drug Discovery™, that leverages evolutionary insights from fungi to unearth next-generation precision medicines. Through our data-immersive and technologically expansive discovery engine,...
Internship
Immediate start
...Job: Drug Safety Specialist
Hybrid- 3 days minimum will be onsite.
The Drug Safety Specialist will have primary responsibility... ...operations, medical information, regulatory affairs, product development, quality assurance, biostatistics, data management, and legal....
Contract work
...Job Description
About This Role
The Medical Director, Drug Safety is responsible for global pharmacovigilance for marketed... ...causality.
Provide safety strategic leadership for clinical development programs
Integrate the safety scientific component to building...
Local area
Worldwide
...Senior Drug Safety Specialist, Compliance - Contract - Cambridge, MA
Proclinical is on the lookout for a dedicated and experienced... ..., supporting records management and compliance across clinical development and marketing programs. This role is crucial in ensuring that...
Contract work
...Executive Director, Drug Safety & Pharmacovigilance
About the Company
Innovative biopharmaceutical company
Industry
Pharmaceuticals
Type
Public Company
Founded
2015
Employees
51-200
Categories
Pharmaceuticals
Medicine
Manufacturing...